News > Is CRISPR safe? Genome editing gets its first FDA scrutiny
Is CRISPR safe? Genome editing gets its first FDA scrutiny
29/10/2023 08:57 AM | Click to read full article
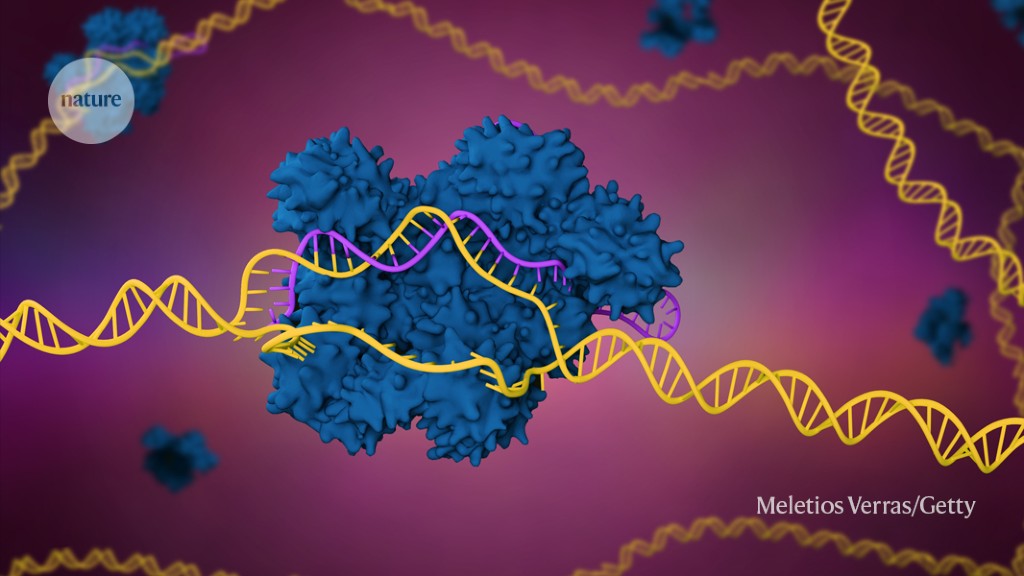
A therapy based on the CRISPR-Cas9 genome editing system could become the first of its kind to gain approval from the US Food and Drug Administration. The treatment, designed to alleviate a painful blood condition, must first face intense scrutiny by the agency and its advisors. On 31 October, external advisors to the FDA will meet to discuss a DNA-altering therapy for sickle-cell disease, a genetic condition that can cause misshapen blood cells and sometimes debilitating pain.





